Expert Guide to the Cleaning and Disinfection of Endoscopes for Healthcare Facilities
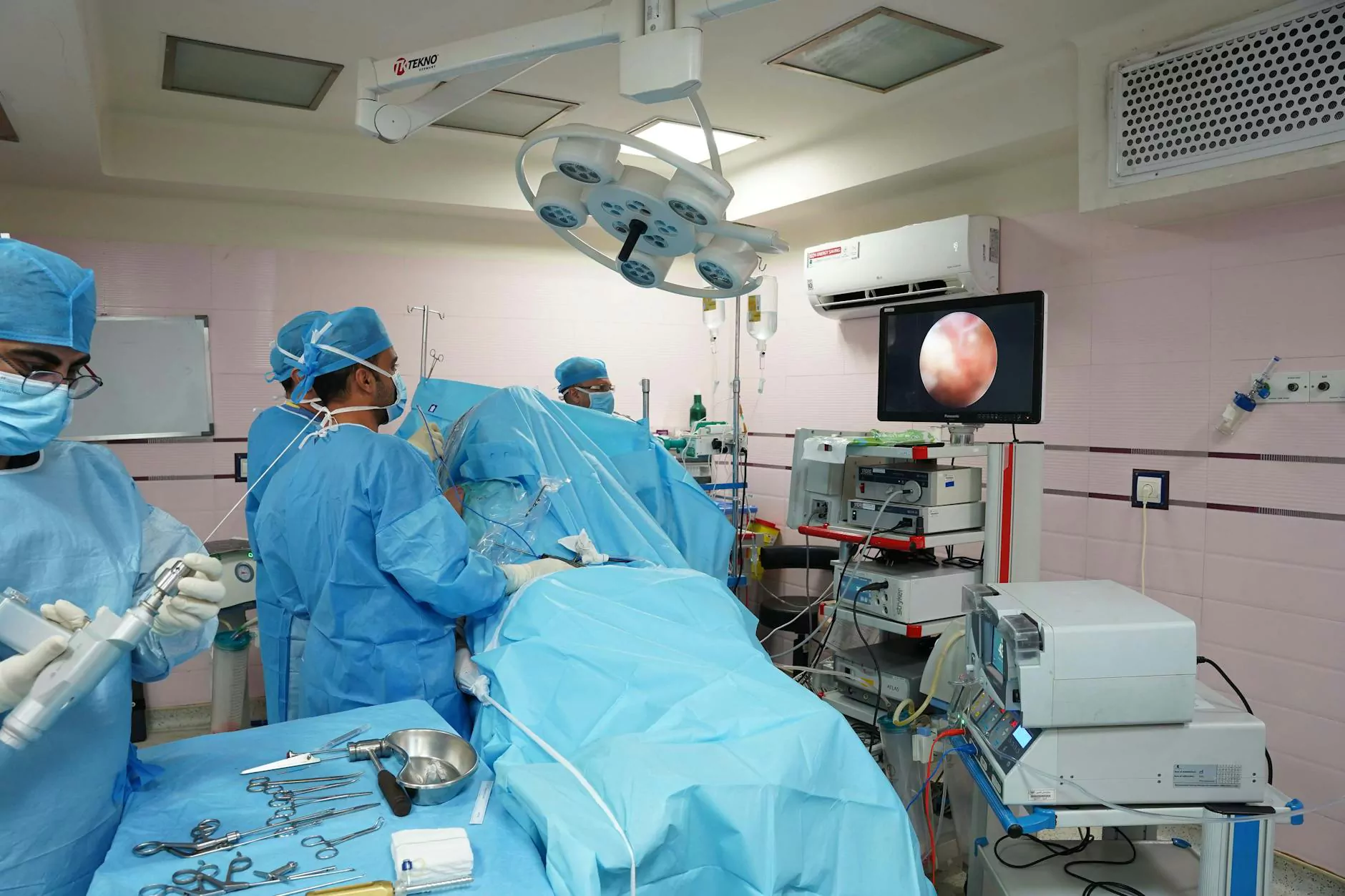
In the realm of healthcare, medical endoscopy plays a pivotal role in diagnosing and treating a myriad of medical conditions. As an essential procedure, it emphasizes the utmost importance of stringent cleaning and disinfection protocols. Proper maintenance of endoscopes not only ensures the safety and well-being of patients but also preserves the integrity and longevity of these sophisticated devices. In this comprehensive guide, we delve into the critical aspects of cleaning and disinfection of endoscopes, illustrating why it is an indispensable part of healthcare operations and how industry-leading practices can optimize outcomes.
Understanding the Significance of Proper Cleaning and Disinfection of Endoscopes
Endoscopes are complex medical instruments that come into direct contact with internal body tissues and fluids. This makes their thorough cleaning and disinfection vital to prevent the transmission of infectious agents such as bacteria, viruses, and fungi. The consequences of inadequate reprocessing can be severe, leading to healthcare-associated infections (HAIs), patient safety risks, and legal liabilities for medical institutions.
Key reasons why proper cleaning and disinfection are crucial include:
- Preventing cross-contamination between patients
- Ensuring compliance with international safety standards and regulations
- Maintaining the highest quality of diagnostic and therapeutic procedures
- Extending the lifespan of delicate endoscopic equipment
Fundamental Principles of Endoscope Reprocessing
Successful reprocessing of endoscopes depends on adherence to established principles and protocols. The core steps include pre-cleaning, manual cleaning, high-level disinfection, rinsing, drying, and proper storage. Each stage must be performed meticulously to eliminate bioburden and prevent microbial growth.
Pre-cleaning
Performed immediately after the procedure, pre-cleaning involves wiping the exterior and flushing lumens to prevent drying of biological debris, facilitating subsequent cleaning efforts.
Manual Cleaning
This critical step involves meticulous scrubbing with approved detergents and brushes to remove organic material and biofilms. Experts emphasize the importance of using validated enzymatic detergents and following manufacturer instructions.
High-Level Disinfection
Using chemical disinfectants that effectively destroy all microorganisms except bacterial spores, high-level disinfection is mandatory for flexible endoscopes. Technologies like automated endoscope reprocessors (AERs) ensure consistent application, but manual disinfection remains vital where automation is unavailable.
Rinsing and Drying
Thorough rinsing with sterile or filtered water removes residual chemicals, followed by drying to prevent microbial proliferation. Special attention should be given to lumens and channels to eliminate residual moisture.
Storage
Endoscopes should be stored in a clean, dry, and well-ventilated environment. Proper hanging or protective covers are recommended to prevent contamination.
Innovative Technologies in Cleaning and Disinfection of Endoscopes
The industry has witnessed significant advancements aimed at enhancing safety and efficiency, including:
- Automated Endoscope Reprocessors (AERs): Devices that automate the cleaning and disinfection process, ensuring consistency and reducing human error.
- Ultraviolet (UV) Disinfection: UV-C light systems are increasingly used to supplement traditional methods, providing rapid sterilization.
- Single-Use Endoscopes: Disposable devices eliminate cross-contamination risks associated with reprocessing.
- Detergent and Disinfectant Innovations: Environmentally friendly, effective solutions designed to eliminate resistant biofilms and shorten processing times.
Implementing these technologies can dramatically improve the safety profile of endoscopic procedures and facilitate compliance with health regulations.
Regulatory Standards and Guidelines for Endoscope Reprocessing
Healthcare providers must adhere to stringent regulations issued by agencies such as the Centers for Disease Control and Prevention (CDC), European Society of Gastroenterology, and local health authorities. These guidelines provide detailed instructions on cleaning, high-level disinfection, sterilization, and storage protocols.
Key compliance aspects include:
- Regular staff training and certification
- Validation and routine maintenance of reprocessing equipment
- Documentation of all reprocessing procedures
- Monitoring microbial burden through periodic testing
- Audit and quality assurance programs
Prioritizing compliance not only reduces risk but also enhances the institution’s reputation and operational efficiency.
Best Practices for Effective Cleaning and Disinfection
To maximize safety and effectiveness, consider these expert-recommended best practices:
- Immediate pre-cleaning: Initiate cleaning promptly after procedure completion.
- Use validated cleaning agents: Select enzymatic detergents and disinfectants approved by regulatory authorities.
- Follow manufacturer instructions meticulously: Ensure each equipment manufacturer’s specific recommendations are adhered to.
- Employ trained personnel: Regular training programs should be in place for staff members involved in reprocessing.
- Implement routine quality checks: Regular microbial testing and equipment validation safeguard process integrity.
- Maintain comprehensive documentation: Record all cleaning, disinfection, and maintenance activities for accountability and audits.
Role of Medalkan in the Medical Supplies Industry
Medalkan stands out as a trusted provider specializing in Health & Medical Supplies, including high-quality products for endoscope reprocessing. Our extensive catalog features:
- Validated enzymatic detergents designed for effective biofilm removal
- High-grade disinfectants complying with global safety standards
- Automated endoscope reprocessing systems to streamline workflows
- Storage solutions that promote contamination-free environments
Partnering with Medalkan guarantees access to innovative, reliable, and compliant products that support healthcare providers' commitment to patient safety and quality care.
Conclusion: Elevating Healthcare Standards with Effective Endoscope Reprocessing
In conclusion, cleaning and disinfection of endoscopes is an indispensable component of modern healthcare practice. Ensuring meticulous adherence to protocols, leveraging cutting-edge technologies, and choosing quality supplies from trusted vendors like Medalkan can significantly reduce infection risks, enhance procedural outcomes, and maintain compliance with rigorous standards.
By prioritizing these practices, healthcare facilities can foster a culture of safety, efficiency, and excellence—ultimately leading to better patient outcomes and a stronger reputation within the medical community.
For comprehensive solutions and expert guidance on medical supplies related to endoscope reprocessing, visit medalkan.com. Together, we can advance healthcare safety and innovation for a healthier tomorrow.









